- We significantly reduce trial timelines by leveraging industry-leading program management to ensure execution predictability.
- We accelerate patient recruitment and sample collection by utilizing our preferred relationships with multi-site research networks.
- We ensure that all success metrics are met by capitalizing on AI-backed analytics to monitor trial progress and proactively adjust to minimize deviations.
Driving Precision in Diagnostics Through Purpose-Built Clinical Research
Your dedicated diagnostics support
We focus on what truly matters: delivering tailored, ICH-GCP-compliant, regulatory-aligned solutions that accelerate your diagnostic product’s path to market with clarity, confidence, predictability, and speed.

Fast.
Predictable.
Smart.

Every study we lead is conducted in full accordance with the International council on Harmonisation of Good Clinical Practice (ICH GCP) E6(R3) guidelines, ensuring ethical standards, data integrity, and regulatory readiness across all phases of research.
Accelerating Tomorrow's Diagnostics.
Accelerating Tomorrow's Diagnostics.
Accelerating Tomorrow's Diagnostics.


Ensuring reliable clinical execution for your diagnostic trials.
The diagnostics industry is undergoing a profound transformation, powered by breakthroughs in molecular diagnostics, artificial intelligence, and precision medicine. As the market expands, so does the number of CROs competing for attention. But in this race for scale, the essence of a truly client-focused partnership is too often lost.
At Dynamill Research, we bring the focus back to what matters most: YOU.
This focus is magnified by transparent ways of working to ensure that sponsors have end-to-end, real-time visibility—enabling faster, risk-based decision-making that leads to greater speed and predictability of results. Furthermore, in this evolving landscape, regulations are becoming more complex by the day and require a vigilant and critical eye for smooth navigation and pathway assessments. This is one of our core areas of expertise, and we take pride in it. It also translates into higher predictability of trial results and accelerates the trial process, resulting in time and cost savings for you.
Our approach is rooted in scientific precision, regulatory fluency, expedience, and personalized collaboration. From early planning to trial execution, we align every step of the process with your product’s pathway, accelerating development without compromising quality or compliance.
Predictability and speed are at the core of how we operate. From patient onboarding to final deliverables, our streamlined processes are built to reduce variability, accelerate timelines, and ensure consistently accurate outcomes—so you can plan with greater certainty and move forward with confidence.
Success finished crative projects with help AI
0
+
Our Value Proposition:
Trial execution predictability
Pre – Trial Activities
Concept Development:
- Understanding Client Needs
- Stakeholder engagement (clinicians, statisticians, regulatory)
Concept Development:
- Protocol development
- Ethics and compliance
- Cost and resource analysis
Site Selection and Setup:
- Site identification
- Site qualification
Regulatory Submissions:
- Prepare documents (IDE, IRB etc.)

Trial Executions
Concept Development:
- Patient identification
- Informed consent
- Sample collection
Study Implementation:
- Test execution
- Comparator testing
- Data collection and recording
- Monitoring
Database Management:
- Database setup
- Data entry
- Quality checks

Post Trial Activities
Data Analysis:
- Statistical evaluation
- Subgroup analysis
- Comparative analysis
- Address anomalies/outliers
Regulatory Decision:
- Adjust and rerun/reanalyze

Customized trial designs
- We design trials specifically tailored for diagnostic validation, including sensitivity and specificity assessments, real-world usability studies, and navigation through complex regulatory pathways.
Pre – Trial Activities
Study Design:
- Ethics and compliance
- Cost and resource analysis
Site Selection and Setup:
- Site identification
- Site qualification
Regulatory Submissions:
- Prepare documents (IDE, IRB etc.)

Trial Executions
Recruitment and Enrollment:
- Patient identification
- Informed consent
- Sample collection
Study Implementation:
- Data collection and recording
Database Management:
- Database setup
- Data entry

Post Trial Activities
Data Analysis:
- Subgroup analysis
- Comparative analysis
Regulatory Decision:
- Adjust and rerun/reanalyze

Provide integrated data solutions to enhance trial endpoint success
- We use smart tools that are fully integrated across the trial lifecycle, allowing us to transparently access and provide data.
- Our digitized tools minimize human intervention and errors, enhancing data integrity and subsequently improving trial success predictability.
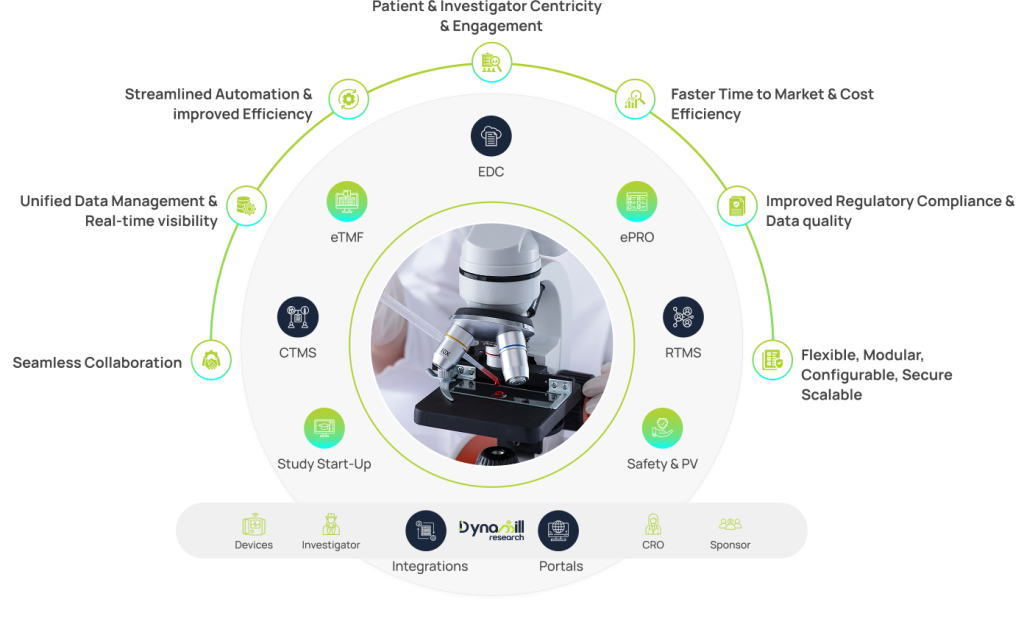
- We use smart tools that are fully integrated across the trial lifecycle allowing us to transparently access and provide the data.
- Our digitized tools minimize human interventions and errors, enhancing the data integrity, subsequently improving trial success predictability.
Pre Trial
Concept Development:
- Regulatory pathway assessment
- Stakeholder engagement (clinicians, statisticians, regulatory)
Study Design:
- Protocol development
- Ethics and compliance
- Cost and resource analysis
Regulatory Submissions:
- Prepare documents (IDE, IRB etc.)

Trial Executions
Study Implementation:
- Monitoring
Database Management:
- Quality Check

Post Trial Activities
Regulatory Decision:
- Adjust and rerun/reanalyze

Provide regulatory expertise and support
We offer specialized teams to navigate complex regulatory environments for better pathway assessment, enhancing trial predictability.
- We offer post-approval support that requires ongoing surveillance until the last mile.
Pre – Trial Activities
Concept Development:
- Understanding Client Needs
- Stakeholder engagement (clinicians, statisticians, regulatory)
Study Design:
- Cost and resource analysis

Trial Executions
Recruitment and Enrollment:
- Patient identification
- Informed consent
- Sample collection
Study Implementation:
- Data collection and recording
Database Management:
- Database setup

Post Trial Activities
Data Analysis:
- Statistical evaluation
- Address anomalies/outliers
Regulatory Decision:
- Adjust and rerun/reanalyze

Utilize strong Site network and SMO partnerships.
- With a vast site network and SMO partnerships, our integrated tools enable us to conduct Decentralized Clinical Trials (DCTs) increasing patient accessibility and diversity by eliminating geographical and logistical barriers
Pre – Trial Activities
Concept Development:
- Understanding Client Needs
- Regulatory pathway assessment
- Stakeholder engagement (clinicians, statisticians, regulatory)
Study Design:
- Protocol development
- Cost and resource analysis
Site Selection and Setup:

- Site identification
- Site qualification
Regulatory Submissions:
- Prepare documents (IDE, IRB etc.)
Trial Executions
Recruitment and Enrollment:
- Patient identification
- Informed consent
- Sample collection
Study Implementation:
- Test execution
- Comparator testing
- Data collection and recording
- Monitoring
Database Management:
- Database setup
- Data entry
- Quality checks

Post Trial Activities
Data Analysis:
- Statistical evaluation
- Subgroup analysis
- Comparative analysis
- Address anomalies/outliers
Regulatory Decision:
- Adjust and rerun/reanalyze

Provide client-focused flexibility
- We provide modular service offerings, allowing clients to choose only the services they need, from regulatory submissions and protocol development to full trial management.
Pre – Trial Activities
Concept Development:
- Understanding Client Needs
- Regulatory pathway assessment
- Stakeholder engagement (clinicians, statisticians, regulatory)
Study Design:
- Protocol development
- Ethics and compliance
- Cost and resource analysis
Site Selection and Setup:

- Site identification
- Site qualification
Regulatory Submissions:
- Prepare documents (IDE, IRB etc.)
Trial Executions
Study Implementation:
- Test execution
- Comparator testing

Post Trial Activities
Data Analysis:
- Address anomalies/outliers
Regulatory Decision:
- Adjust and rerun/reanalyze

Insignts
The Team Behind the Vision
Dynamill Research is led by a cross-functional team of scientific, operational, and regulatory experts who understand the distinct challenges and opportunities of diagnostic trials. From molecular assay validation to complex multi-site studies, our team brings both a strategic perspective and day-to-day executional excellence.

Dr. Murtaza HussainOperations Advisor

Joseph CohilC.C.O. (Chief Commercial Officer)

Mohammad MilwalaS.M.O. Partner

Talal KhanHead of Scientific & Clinical Affairs

Aqsa ZamanHead of Quality

Talal KhanHead of Scientific & Clinical Affairs

Bilal PatelHead of Marketing

Aqsa ZamanHead of Quality

Joseph CohilC.C.O. (Chief Commercial Officer)

Aqsa ZamanHead of Quality

Bilal PatelHead of Marketing

Dr. Murtaza HussainOperations Advisor

Talal KhanHead of Scientific & Clinical Affairs
How We Think
Every trial we design is guided by the realities of modern diagnostics, such as high data volume, complex endpoints, urgent timelines, and regulatory intricacies.
That’s why we’ve built Dynamill Research to be agile, integrated, and accountable. We operate as an extension of your team—focused not just on getting trials done, but on getting them done right.
We don’t generalize. We don’t overcomplicate.
We partner, we execute, and we deliver.
Contact Us
![]() +1877- DYNAMILL (3962 6455)
+1877- DYNAMILL (3962 6455)
Our Location
![]() 16W285 83rd St. STE D-1 Burr Ridge, IL 60527
16W285 83rd St. STE D-1 Burr Ridge, IL 60527
Email
![]() info@dynamillcro.com
info@dynamillcro.com
Social Network
16W285 83rd St. STE D-1 Burr Ridge, IL 60527